Amazon.com: Cyber Nickel Copper 1 Faraday Fabric EMF Shielding 50" Width Signal Blocking Material - Plain Weave… (50" x 3')

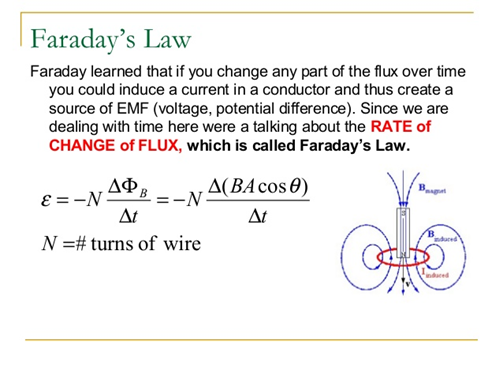
FARADAY'S LAW. NaCl (s) → Na + (l) + Cl – (l) E° R = V E° O = V 2 Cl - (l) → Cl 2(g) + 2e - 2 Na + (l) + 2e - → 2 Na (s) Electrolytic cell. - ppt download
the amount of copper deposited by 1 faraday current will be maximum in an acidic solution of 1l of 1.1MCu2Cl2 2.2MCu(NO3)2 3.5M CuSO4 4.5M Cu3(PO4)3 5.10M CuF2

The mass of the substance deposited by 1 faraday of electricity is equal to 11 grams. The value of electrochemical equivalent is: A. 11 B. 11 x 96500 - Correct Answers 11 96500 D. data insufficient